Introduction
The Cleveland FES Center fabricates prototype implantable research devices that are intended for use in human feasibility studies conducted under several Investigational Device Exemptions (IDEs). In 1991, the Technical Development Laboratory (TDL) was established to facilitate the design and development of implantable hardware required for clinical studies within the Cleveland FES Center.
The Technical Development Laboratory (TDL)
The TDL is a resource core for the FES community that provides design, development, fabrication, and testing of implantable and external systems, instrumentation, and software. Technical capabilities include system integration, electronics design, multi-level software design, mechanical design, material science, and project management. The TDL facilities include the cleanroom, micro-fabrication lab, machine shop, electronics lab, embedded systems lab, computer lab.
Organization and Control of Human Implantable Device Design
To facilitate the transfer of promising technology to the commercial realm, the TDL has implemented Standard Operating Procedures (SOPs) that define and delineate activities related to 21 CFR 820 Subpart C, Design Controls. The ongoing clinical studies within the Cleveland FES Center are conducted under Investigational Device Exemptions (IDEs), which are governed by 21 CFR 812.
Facilities
The TDL’s micro-fabrication lab is a materials facility used for prototyping and analysis as well as fabrication of components that don’t require the rigors of a cleanroom. The lab includes several types of microscopes that include video and photographic documentation capabilities, circuit board screen printer, a spot welder, soldering/de-soldering workstations, balances, several ovens including a vacuum oven, a surface-mount technology (SMT) flow oven, furnace, fume hoods, and a water bath.
The TDL’s electronics lab provides workstations for bench top development and testing, GPIB networked instrumentation including oscilloscopes, bench meters, power supplies, a spectrum analyzer, an RF power meter, arbitrary waveform generators, and custom built instrumentation. The electronics lab maintains an extensive inventory for fabricating prototypes as well as specialized SMT rework stations. Within the electronics lab is the embedded systems lab, which provides software and hardware tools such as compilers, assemblers, linker/loaders, emulators, background debuggers, and a revision control system. A computer modeling lab provides workstations for CAE/CAD/CAM, modeling, rapid prototyping, and simulation.
TDL has a machine shop that includes a three-axis CNC milling machine, circuit board prototyping system, bead blaster, diamond dicing saw, lathes, a conventional milling machine, a band saw, a surface grinder, a jeweler’s lathe, drill presses, and a belt/disk sander.
Expertise/Core Competencies
Over the past 25 years, our effective use of the core competencies has enabled the TDL to develop, implement, and transfer to industry numerous implanted hardware components, external hardware components, and integrated software solutions focused on implantable pulse generators for restoration of function in individuals paralyzed by stroke or SCI.
There are five core areas of expertise:
- External hardware design and development
- Implanted hardware design and development
- Quality Systems management
- Project management/product development
- Technology Transfer
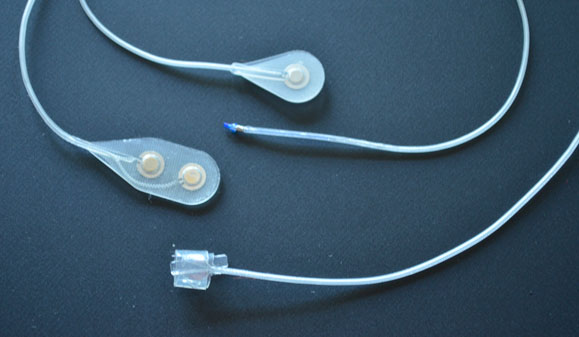
Four types of human-quality implantable electrodes (from top: epimysial stimulating electrode, intramuscular stimulating electrode, epimysial recording (bipolar) electrode, spiral nerve cuff electrode). All electrodes were designed, developed and fabricated within our facility. Depending on the application, electrodes can be fabricated for mono-polar or bi-polar use.
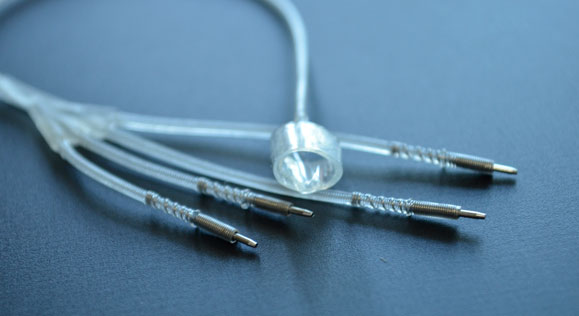
Spiral nerve cuff electrode in four-contact/quad lead configuration. This electrode is self-sizing and wraps around the epineurium to activate the target nerve entirely or to activate individual fascicles within the nerve bundle. This electrode was designed, developed and fabricated within our facility. Depending on the application, these electrodes can be fabricated for mono-polar or multiplexed use.

From left, epimysial stimulating electrode (monopolar), and epimysial recording (bipolar) electrode. This electrode is sutured to the muscle epimysium near the target motor point (for stimulation) or near the best electromyogram (EMG) site (for recording). These electrodes were designed, developed and fabricated within our facility.
We have the facilities and capabilities to take your idea from concept to reality. We can help you demonstrate feasibility or to build devices which will enable clinical data collection for study validation.
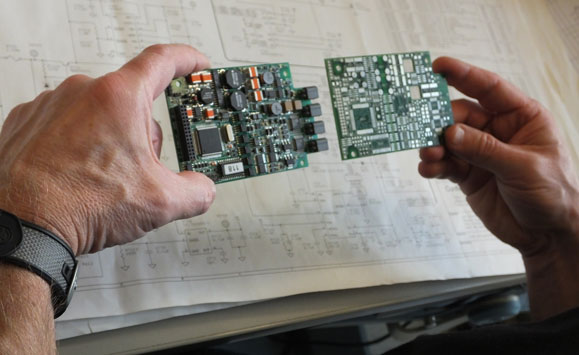
One of our core competencies is in the design, layout, fabrication, assembly and design verification of circuit boards and/or electronic hardware for your idea.
Publications
Contact
James P. Uhlir, MBA
Operations Manager
Case Western Reserve University
Bingham Building, Room 306
2104 Adelbert Road
Cleveland, Ohio 44106
(216) 368-3153; Fax (216) 368-3115
jpu2@case.edu
