Grasp-Release Assessment of a Networked Neuroprosthesis Device (GRANND)
The goal of this clinical trial is to improve upper extremity function in people with cervical-level spinal cord injury (SCI). Specifically, this study is testing the effectiveness of an implanted networked neuroprosthesis device to improve the ability to grasp and release objects.

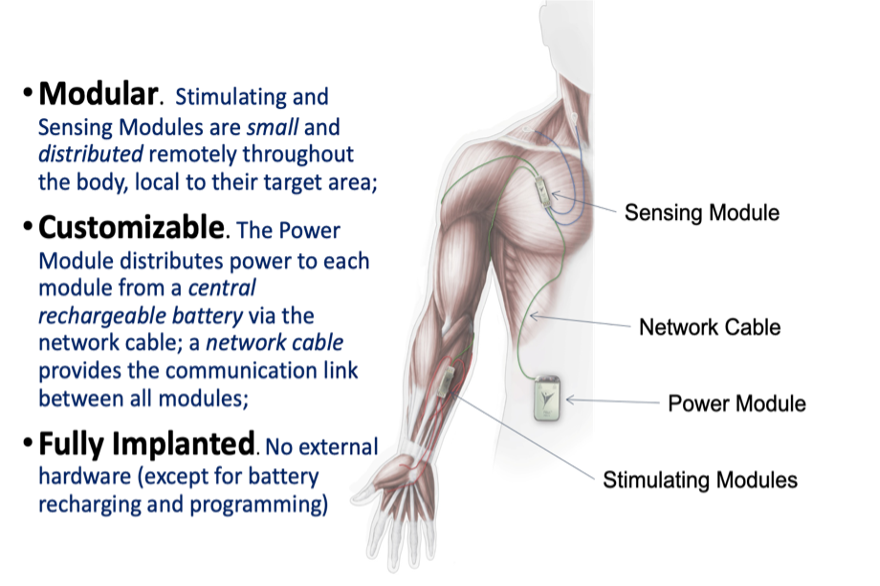
What’s a Networked Neuroprosthesis Device?
The networked neuroprothesis device (NNP) involved in this study is a surgically implanted functional electrical stimulation device that restores function in the paralyzed hand. Individuals use muscles under their voluntary control to “turn on” and “turn off” grasp patterns to manipulate various objects.
What’s Involved?
Prior to implantation, prospective participants will be evaluated for study and surgical candidacy. This may involve direct or coordinated efforts with a participant’s healthcare team to ensure safe enrollment. Surgical implantation involves hospital admission, and participants surgical arm will be placed in a cast to protect the newly implanted device. The cast will remain on for three weeks. Participants may return home during this period or stay in the Metrohealth Center for Rehabilitation Research (MCRR) Zubizarreta House (see details below). Next, the cast is removed and the implanted devices will be programmed with our research team to begin exercising using their new arm movement. Five weeks following this, participants will be trained on integrating their new arm movements into functional daily activities. Outcome measures will be obtained prior to surgical implantation, and again at 3, 6, and 12 months.
Am I a Candidate?
If you are living with cervical-level SCI sustained as least 6 months ago, medically stable, and at least 17 years old, you may be a candidate for this study. Prior to enrollment, prospective participants will be evaluated to determine the electrical excitability of their upper extremities to ensure favorable response to implantation of the device.
Where Can I Stay?
Long distance participants and their families (and/or caregivers) will have access to the Zubizarreta House, a 7,000 square foot accessible living space adjacent to MCRR at the Metrohealth Old Brooklyn Campus. The house provides private suite rooms with track lifts, low pressure beds, private roll-in showers, and separate caregiver rooms. On-site laundry, and a common kitchen and living area are available to guests.
Detailed program information and criteria available at: https://clinicaltrials.gov/study/NCT05863754#study-overview
Principal Investigators: Kevin Kilgore, PhD; Anne Bryden, PhD, OTR/L; Megan Moynahan, MS
Program Contact: Kimberly Walsh, OTR/L
Contact Number: (216) 957-3512
Contact Email: kwalsh3@metrohealth.org
Contact Request
Researchers rely on individuals to serve as volunteers for program studies. Each study is designed to answer questions about a specific medical aspect or the effectiveness of a particular treatment. Through the commitment of research volunteers, knowledge gained and communicated to other medical professionals ultimately benefits the community.
If you would like more information about becoming a research volunteer please submit the information below.
