A Research Focus on Neuromodulation
We Study the Use of Functional Electrical Stimulation to Improve, Treat or Reverse Neurological Symptoms
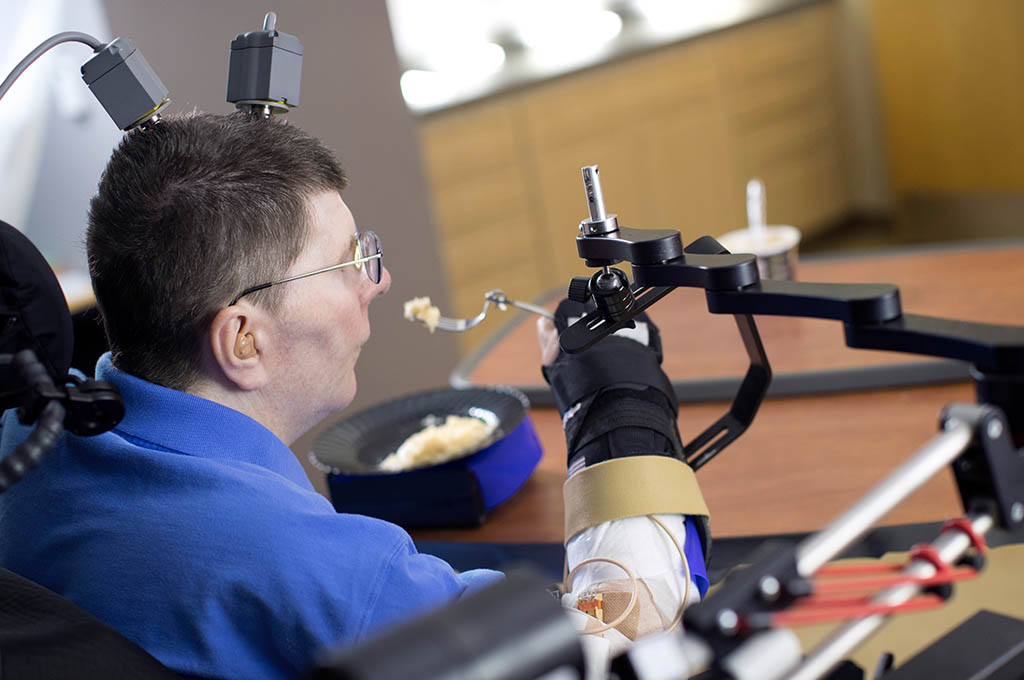
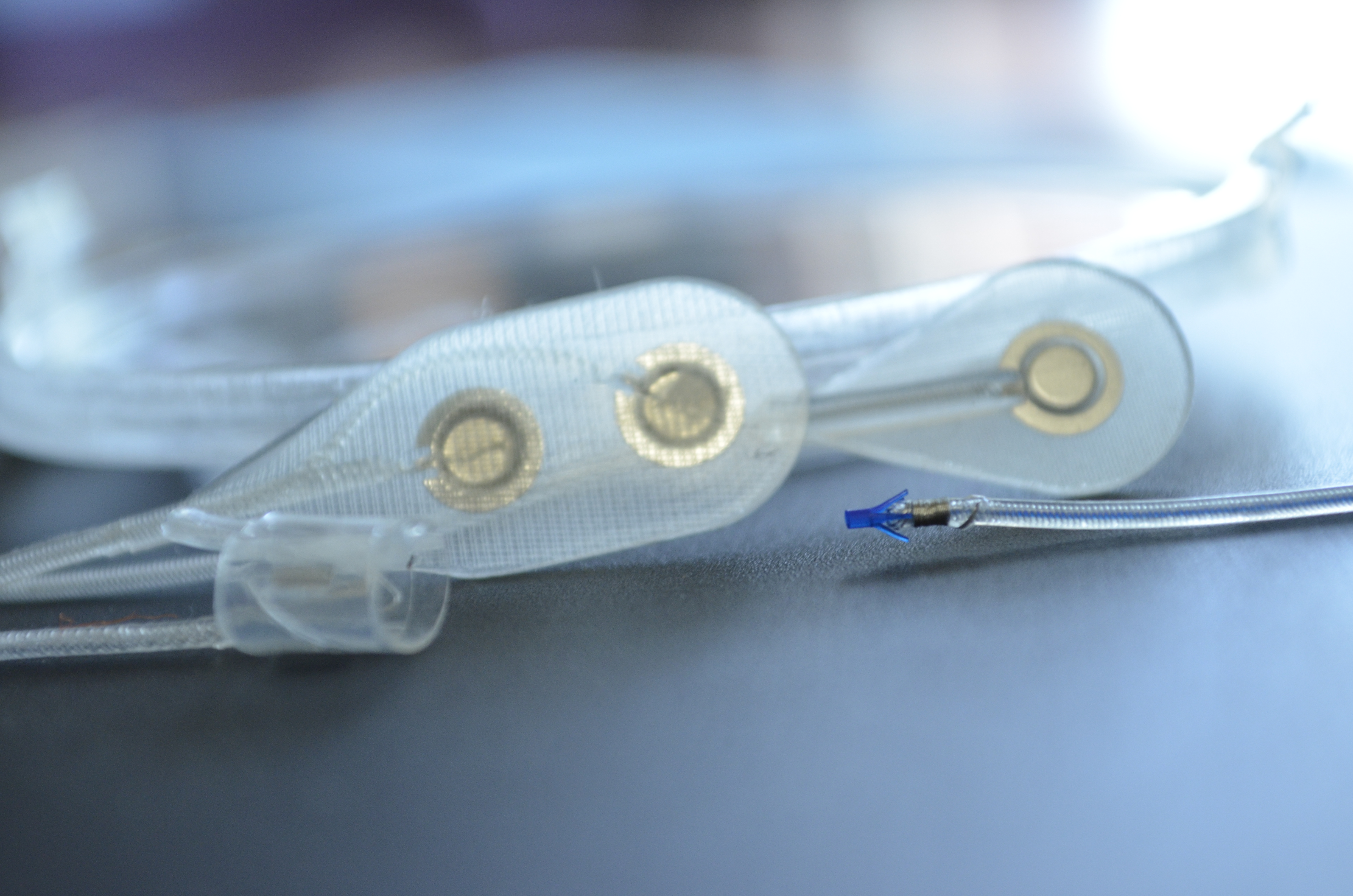
Functional electrical stimulation (FES), or neuromodulation, is the use of small, artificially generated low-level electrical pulses that are safely and selectively applied to the central or peripheral nervous system to replace the actions of neurons that have been damaged by injury or disease.
When applied appropriately, FES can “speak the language of the nervous system” and evoke desired actions by both activation and inactivation of various elements of the nervous system (e.g., peripheral nerves, spinal cord, brain).



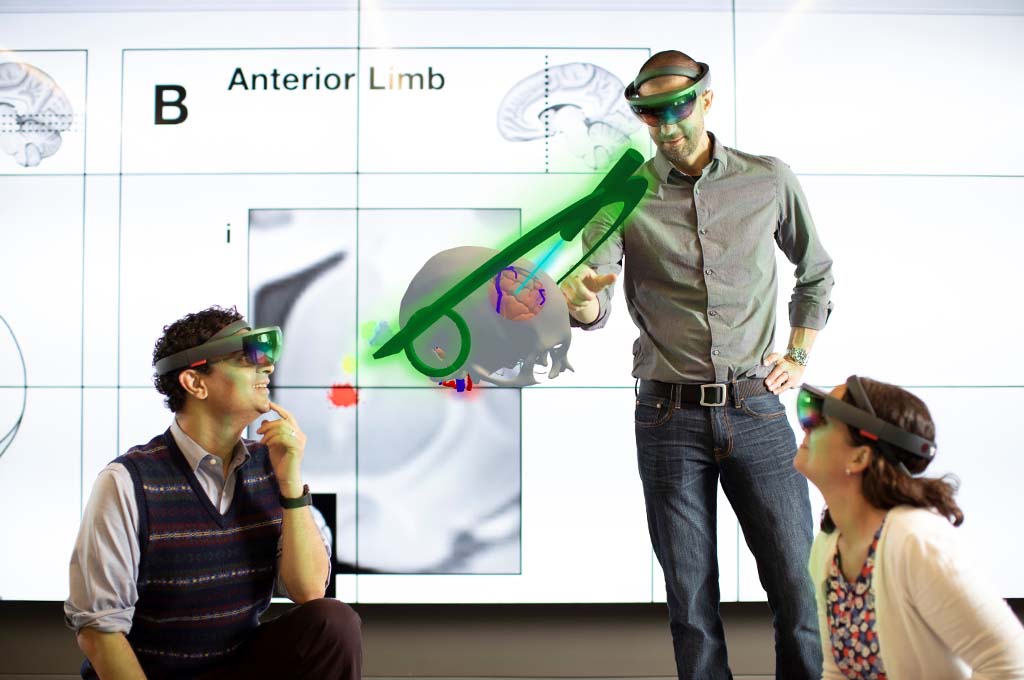
The FES Center is the most comprehensive and cohesive program in the world
performing FES investigation that spans from basic to applied,
and our investigators work on many different applications.